Depending on the amount of baking soda added to magnesium, how much do the bubble rise?
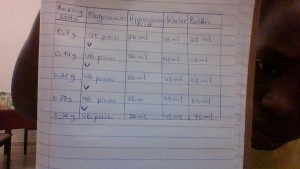
IV: Amount of baking soda put in magnesium
DV: The rise of the bubbles
Controlled Variables: Amount of baking soda, magnesium, water, soap.
Hypothesis {BEGINNING}: The more magnesium will add the lower the bubbles will rise.
DATA TABLE
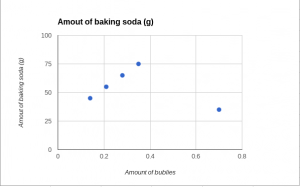
SCATTER PLOT
Discussion: How did it go? What worked with the experiment? What would you improve next time?
How did it go: Overall, The experiment was a success even if we had to restart once the experiment. We made a mistake and put the baking soda in the water rather than the acid.
What worked with the experiment: Everything worked fine{ the measurements} except for the soap because we put a little bit too much soap, it was hard to decide the right number of bubble rise on the tube.
What would you improve next time: Personally I think that if my partner listened to my ideas, we wouldn’t have to restart. I would like to improve the communication between me and my partner.
Based on my data, I can conclude that… Our bubbles went up by 10 ml each time and also the about of baking soda, also increased by 7.
Based on my data, I can conclude that… The bubbles went up by 10 ml each time we added baking soda (we added 7 grams of baking soda more than the previous.)